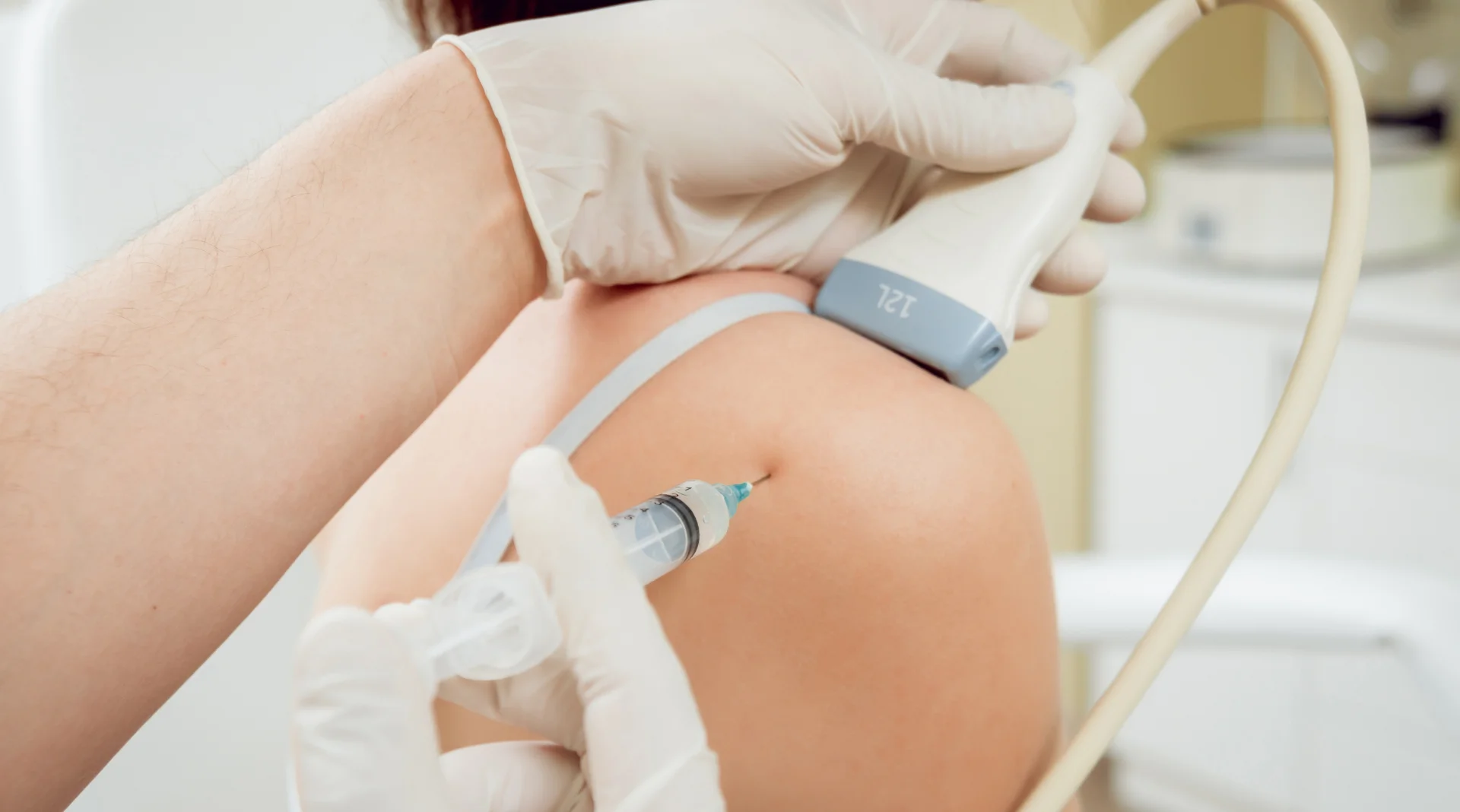
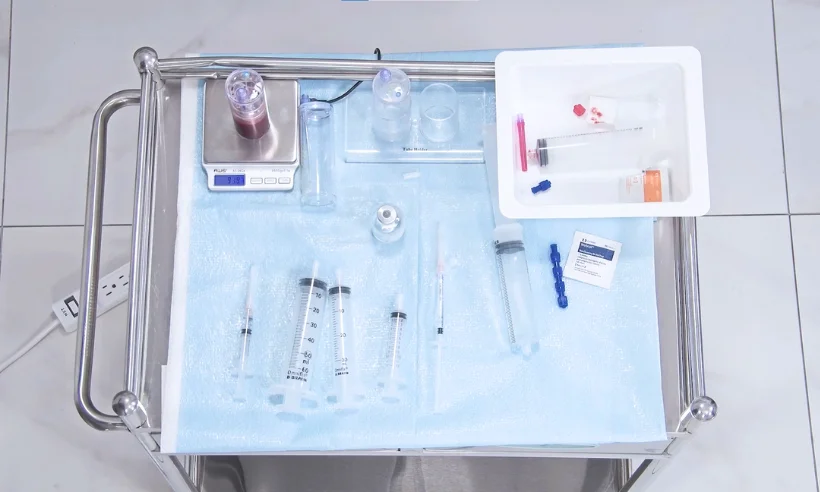
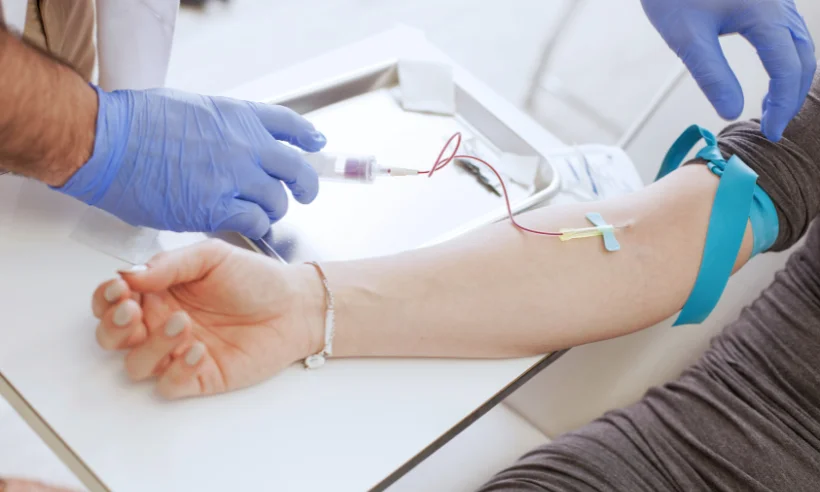
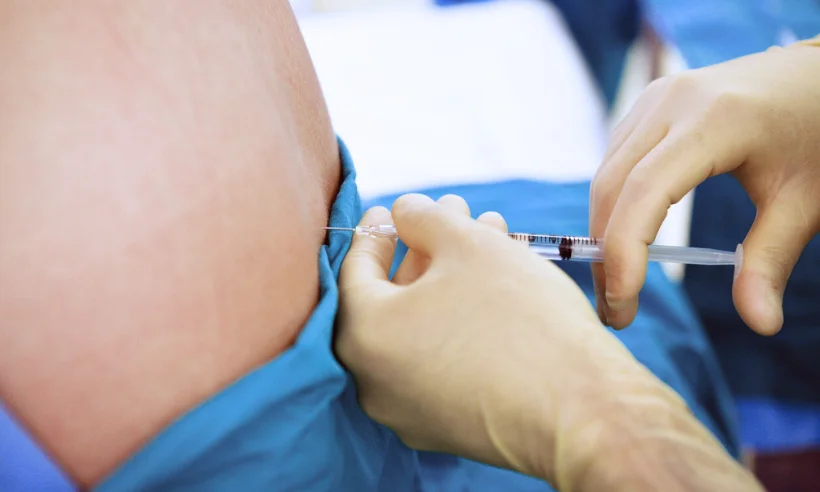
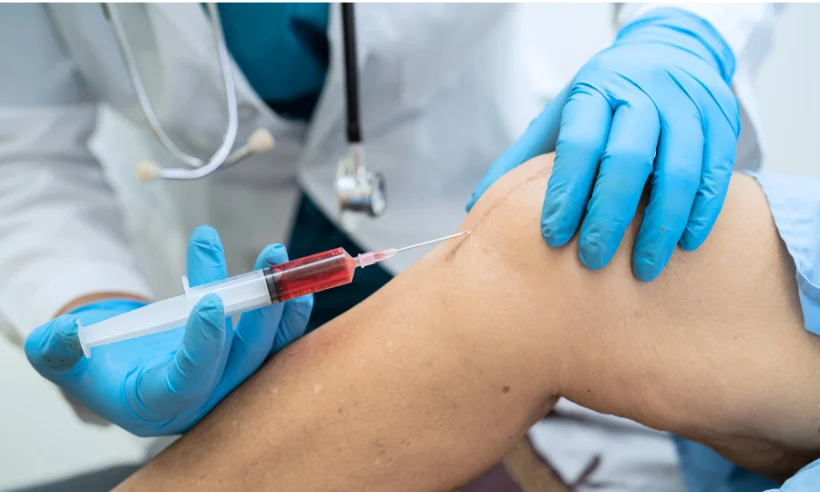
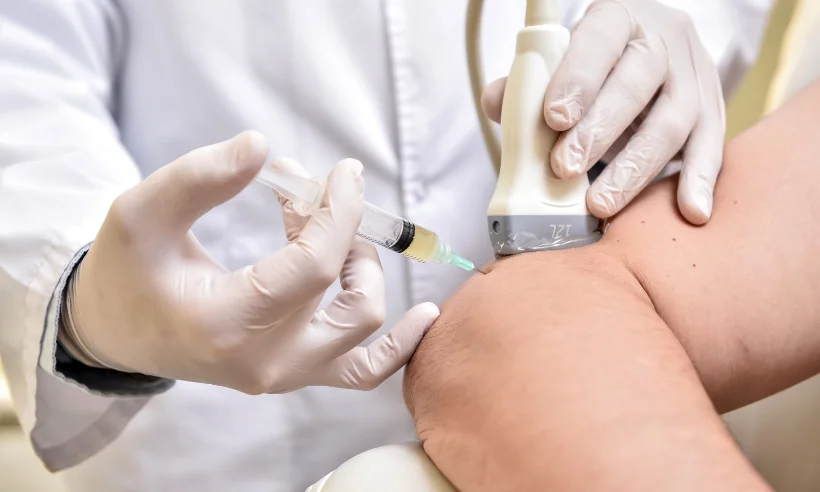
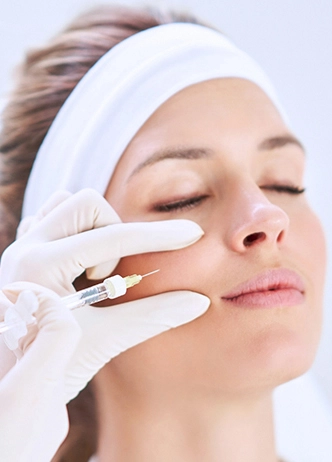
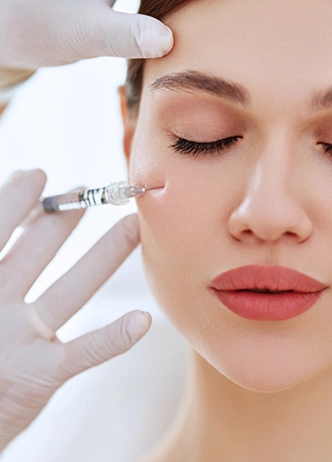
Included in the course study will be concentrated growth factors to enhance your ACP injections using allograft concentrated growth factors and stem cells from amniotic tissue or bovine (A-Cell).
Common medical uses and indications for the use of ACP and Allograft CGF (Concentrated Growth Factors) include:
-
Shoulder Area
- Rotator Cuff (Partial Tear)
- Biceps Tendinosis
- Glenohumeral Ligament Sprains
- Shoulder Joint (Arthritis Pain)
-
Elbow Area
- Epicondylitis
- Distal Biceps Tendon (Partial Tear)
- Elbow Joint (Arthritis Pain)
-
Hand Area
- Chronic Thumb UCL Sprain
- Small Digit Injections (Arthritis Pain)
-
Knee Area
- Patellar Tendinosis
- Meniscal Tears (Partial Tear)
- Vastus Lateral Oblique Strains (Chronic)
- Osgood-Schlatters
- Knee Joint (Arthritis | Cartilage Regeneration)
-
Spinal Area
- Para-Spinal Muscles (Failed Back Surgery)
- SI Joint Injections (Increased Vascularity)
-
Ankle | Foot Area
- Chronic ATF Ligament Strains
- Chronic Achilles Tendinosis
- Chronic Peroneal Tendon Tears
- Plantar Fasciitis
-
Other Medical Conditions
- Diabetic Neuropathies (Lower Extremities – Ischemic)
- Carpal Tunnel Syndrome (Scar Tissue)
- Jones Fracture (Accelerated Healing | Vascular)
- Muscle Atrophies and other soft tissues.
ACP TRAINING LATEST TREATMENT OPTIONS FOR PAIN
Give your patient the latest treatment options for pain, enhance patient care in the management of various pain conditions presented, and differentiate yourself from others with these expanded pain management procedures. The additional revenue streams associated with these procedures are attractive to physicians and are the perfect addition to any medical practice treating these various chronic soft tissue and joint-related arthritis conditions.
As part of the course study, you will be introduced to the various stem cell therapies also being performed in conjunction with ACP or conventional medicine. The growth in using mesenchymal cell therapies is expanding beyond orthopedic offices and the demand from patients is becoming popular. These experimental therapies are being used successfully globally and within the United States but do have additional limitations as these are not FDA-Approved and their uses are limited to orthopedic conditions for larger joint pain. Adipose Stem Cells (ASC) and Bone Marrow Stem Cells (BMAC) are the available options for physicians but there are no professional medical guidelines for who can and cannot receive stem cell therapy. Stem Cell Therapies are considered to be experimental by the FDA so the decision about who gets stem cell therapy is up to patients and doctors and there are legal requirements as to the advertisement of such within your practice.
The Adipose Stem Cell Therapies (ASC) and Bone Marrow Stem Cells (BMAC) procedures include all protocols for harvesting and re-injection, patient selection criteria, contraindications, and expected results for the most common types of arthritis to the knee and shoulder areas. Candidates for such treatment are usually mild to moderate degeneration of capsule and the most robust response to these treatments are candidates that are younger to middle age and in good medical health. There are other experimental uses of mesenchymal cell therapies such as Multiple Sclerosis and Alzheimer’s through the course will concentrate only on the pain management application therapies.
Special Considerations
Give your patient the latest treatment options for pain, enhance patient care in the management of various pain conditions presented, and differentiate yourself from others with these expanded pain management procedures. The additional revenue streams associated with these procedures are attractive to physicians and are the perfect addition to any medical practice treating these various chronic soft tissue and joint-related arthritis conditions.
Empire Medical Training will prepare you to integrate these new procedures within your practice and apply the latest standard of care considerations to be able to perform these safe and effective non-surgical procedures. We provide a large amount of supplemental data for review after the course and attendees will receive complete protocols for all the procedures associated with the ACP (Autologous Conditioned Plasma), Allograft CGF (Concentrated Growth Factors), and Stem Cell Therapies being employed with sports medicine, orthopedic offices, hospitals, and private practice. Marketing, pricing and packaging, and detailed pre and post-treatment options for patients are given as well as the legal, ethical, and administrative concerns associated with the introduction of these new procedures.
All attendees will receive complete injection protocols, standard of care, and complete information on the procedures to diagnose and gauge the improvement of these conditions for treatment.

1-Year Term
In-Person and Virtual Training
Full Certification & Testing
Pre-Course Videos and Training Materials
Medicines (Free Botulinum Toxin, Dermal Fillers, and PDO Threads, etc.)* ($2,100 Value)

1-Year Term + 1 Extra Year FREE
In-Person and Virtual Training
Full Certification & Testing
Pre-Course Videos and Training Materials
Medicines (Free Botulinum Toxin, Dermal Fillers, and PDO Threads, etc.)* ($2,100 Value)

1-Year Term + 1 Extra Year FREE
In-Person and Virtual Training
Full Certification & Testing
Pre-Course Videos and Training Materials
Medicines (Free Botulinum Toxin, Dermal Fillers, and PDO Threads, etc.)* ($2,100 Value)