What Is Botox®?
Neurotoxins | February 13, 2021
What Is Botox®?
Entry made by
Empire Medical Training
BOTOX®, or simply Botox, is an injectable medication indicated and approved for the treatment of a variety of cosmetic and musculoskeletal conditions in adults. Trained clinicians administer millions of Botox doses each year. An
estimate by the American Society of Plastic Surgeons places the total number of annual Botox injections given in the United States ahead of the population of Arizona.
Botox is a drug that has been used in clinical
settings since the late 1970s and for cosmetic indications since the turn of the century. Despite being derived from one of the planet’s deadliest neurotoxins, Botox is safe and effective when administered as indicated by trained
medical or aesthetic professionals.
Of course, prospective Botox treatment candidates have every right to ask questions about where Botox comes from, how it works on the human body, what it really does once injected,
and what to expect from Botox treatment (before, during, and after the procedure itself).
This overview answers those questions and more. It covers:
- The history of Botox in medicine and aesthetics
- How the active ingredient in Botox acts on human muscles
- What Botox does (its cosmetic and therapeutic effects)
- Conditions Botox is indicated and approved to treat (including off-label treatments deemed safe and effective)
- Botox treatment procedures and follow-up
- FDA-approved alternatives to Botox
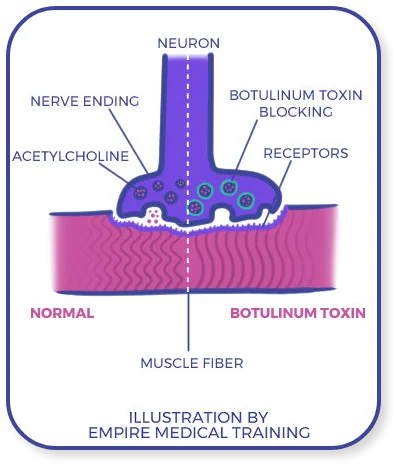
Let’s begin with a detailed look at the scientific advances that made Botox possible and the early history of the medication itself.
History of Botox in Medicine and Aesthetics
The full history of Botox predates its first medical use by nearly a century. The story begins in the late 19th century, when a Belgian bacteriologist discovered the organism responsible for producing its active ingredient.
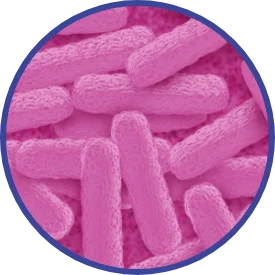
Discovery of Clostridium botulinum
According to an historical retrospective published by the National Institutes of Health, investigation into the causes of botulism — an often
fatal illness most commonly (at the time) associated with consumption of improperly stored sausage — began in the late 18th century. It wasn’t until about 1895, when a botulism outbreak in a Belgian village caught the
attention of University of Ghent bacteriologist Dr. Emile Pierre van Ermengem, that the pathogen responsible for the illness was isolated and described.
That pathogen is a large, rod-shaped bacterium known
as Clostridium botulinum. Gram for gram, some of the neurotoxins it produces — seven main types in all, lettered A through G — are among the deadliest biological products known to science.
Initially, Dr. van
Ermengem’s discovery was hailed as the beginning of the end of what had up until then been an unfortunate and tragic part of human life: occasional outbreaks of deadly foodborne illness caused by botulinum-produced
neurotoxins. The following decades saw a revolution in food production and storage procedures, as well as medical treatments and technologies, that have dramatically reduced the incidence and severity of botulism.
Early Medical Research and Clinical Use of Botulinum Toxin A
Even as Dr. van Ermengem’s work catalyzed a long-overdue food safety renaissance, the scientific community homed in on the possible therapeutic uses of one botulinum toxin in particular: botulinum toxin A, or BoNT-A.
Scientists first isolated botulinum toxin A in crystalline form during the World War II era, but it wasn’t until the 1970s that research into its clinical potential began in earnest. Experimenting first on domesticated poultry
and later on monkeys, ophthalmologists determined that remarkably small volumes of botulinum toxin could induce localized paralysis in extraocular muscles.
The finding offered the tantalizing possibility of nonsurgical
treatment for ocular misalignment (strabismus), a condition that had formerly necessitated invasive and often unsuccessful surgical intervention. Human clinical trials began in the late 1970s and quickly produced clinical data
sufficient to support a thriving ophthalmological niche. Initially known as Oculinum (onabotulinumtoxinA), the medication shortly received FDA approval for the treatment of a second condition: blepharospasm, an abnormal twitch
of the eyelid.
Botox Is Born
By the late 1980s, thousands of North American strabismus and blepharospasm sufferers had come to rely on Oculinum. Sensing commercial opportunity, pharmaceutical giant Allergan applied for and received permission from the U.S. Food and Drug Administration
to market the medication under the brand name “Botox.”
The rebrand coincided with another important development in the science of clinical BoNT-A toxin use: the independent confirmation
by multiple clinicians that Botox was effective in reducing the appearance of facial wrinkles.
The Sacramento-based plastic surgeon Dr. Richard Clark was the first to identify Botox as a promising treatment for forehead
wrinkles in post-surgical patients. He received initial FDA approval for this use in 1989. Meanwhile, in Vancouver, Drs. Alastair and Jean Carruthers discovered that Botox had a welcome side effect for some of their blepharospasm
patients: temporarily reducing glabellar lines, those worried-looking vertical creases between the eyebrows. Reader’s Digest Canada tells the story of their yearslong struggle to convince their colleagues of Botox’s vast potential as a cosmetic treatment.
History proved them right, of course, but they weren’t vindicated overnight. More than ten years passed before
the FDA gave final approval for Botox Cosmetic, a formulation specially designed to treat cosmetic conditions like forehead lines and wrinkles and glabellar lines. (U t point, such treatments were done “off-label” — performed
at clinicians’ discretion in controlled environments but without formal approval from medical regulators.)
Botox Today
Since the turn of the 21st century, the Food and Drug Administration has approved Botox for a host of clinical uses. Medical regulators in dozens of other countries have followed suit, giving rise to a truly global branch of cosmetic and therapeutic medicine. Major FDA approval milestones for Botox include:
- Cervical dystonia, 2000
- Glabellar lines, 2002
- Excessive sweating under the armpits (axillary hyperhidrosis), 2004
- Chronic migraine, 2010
- Upper limb spasticity in adults, 2010
- Urinary incontinence (specific form), 2011
- Crow’s feet, 2013
- Lower limb spasticity in adults, 2016
- Upper and lower limb spasticity in children, 2019
Common off-label uses for Botox include excessive saliva production (sialorrhea), psoriasis and certain types of eczema, certain types of anal dysfunction (anismus), certain types of idiopathic vaginal pain or discomfort (vulvodynia), Raynaud’s disease, and achalasia (difficulty swallowing). As evidence accumulates to support Botox’s use for these issues, some or all may eventually draw formal FDA approval.
A Note About Botox (Botox Therapeutic) and Botox Cosmetic
Allergan markets two distinct Botox brands: Botox, also known as Botox Therapeutic or Botox Medical; and Botox Cosmetic.
In terms of formulation, Botox Therapeutic and Botox Cosmetic are essentially identical. Both
are derived from botulinum toxin A and contain the same toxin concentrations. Both act on human muscles in the same way.
Functionally, Botox Therapeutic and Botox Cosmetic are very different. Botox Therapeutic is marketed
(and indicated) to treat debilitating and/or painful conditions like cervical dystonia and chronic migraine. Botox Cosmetic, by contrast, is marketed and indicated to treat cosmetic issues that don’t directly affect patient
quality of life, such as glabellar lines and crow’s feet.
Common off-label uses for Botox include excessive saliva production (sialorrhea), psoriasis and certain types of eczema, certain types of anal dysfunction (anismus),
certain types of idiopathic vaginal pain or discomfort (vulvodynia), Raynaud’s disease, and achalasia (difficulty swallowing). As evidence accumulates to support Botox’s use for these issues, some or all may eventually draw
formal FDA approval.
A Note About Botox (Botox Therapeutic) and Botox Cosmetic
Allergan markets two distinct Botox brands: Botox, also known as Botox Therapeutic or Botox Medical; and Botox Cosmetic.
In terms of formulation, Botox Therapeutic and Botox Cosmetic are essentially identical. Both
are derived from botulinum toxin A and contain the same toxin concentrations. Both act on human muscles in the same way.
Functionally, Botox Therapeutic and Botox Cosmetic are very different. Botox Therapeutic is marketed
(and indicated) to treat debilitating and/or painful conditions like cervical dystonia and chronic migraine. Botox Cosmetic, by contrast, is marketed and indicated to treat cosmetic issues that don’t directly affect patient
quality of life, such as glabellar lines and crow’s feet.
It’s critical for patients and clinicians alike to understand that Botox Cosmetic is not inherently “safer” than Botox Therapeutic. Both must be administered
by trained clinicians in controlled settings, regardless of the condition being treated. In this context, “trained clinicians” typically refers to licensed physicians, aestheticians, or midlevel providers working under the
supervision of a physician.
Botox Treatment: An Overview
Healthcare professionals should refer to official medication literature, FDA supplemental materials, and continuing medical education curriculum for more detail on injecting Botox and managing Botox treatment candidates. The following is for informational purposes only.
How to Evaluate Botox Treatment Candidates
Every course of Botox treatment begins with an initial patient consultation. During this consultation, clinicians must verify that the patient receiving Botox does not present with any medical issues that contraindicate its use:
- Myasthenia gravis
- Lambert-Eaton syndrome
- Congestive heart failure
- Diabetes (Type 1 or 2)
- Rheumatoid arthritis
- Known sensitivity to any Botox (or competitor) component, including inactive ingredients like albumin, sucrose, or lactose
- Anatomical defects such as facial asymmetry or ptosis
- Known history of facial palsy
- Current or recent skin infection in the treated area
- Current or expected pregnancy or breastfeeding
- Use of certain contraindicated medications, including anticoagulants, aminoglycoside antibiotics, muscle relaxers, and drugs known to interfere with Botox
Diluting and Injecting Botox Safely
Botox comes in five different FDA-approved formulations:
- Botox 50 units/vial
- Botox 100 units/vial
- Botox 200 units/vial
- Botox Cosmetic 50 units/vial
- Botox Cosmetic 100 units/vial
Depending on the procedure, injections of botulinum toxin are generally intradermal or subcutaneous. Take care to avoid overly shallow or deep angles of entry, either of which may reduce treatment efficacy or increase the risk of systemic complications. Likewise, avoid major blood vessels and nerves, and mind site-specific risks and best practices, such as avoiding the area around the upper orbital bone when injecting Botox around the eyes.
What to Do After Botox Treatment: Assessing Results and Monitoring for Complications
Botox side effects, allergic reactions and complications are relatively rare. However, they can occur in any patient after any treatment. Providers must counsel patients to watch for the most serious, which necessitate immediate medical evaluation:
- Difficulty breathing (which can be life-threatening if left untreated)
- Difficulty swallowing (which can also be life-threatening without treatment)
- Muscle weakness (in specific muscle groups or generalized throughout the body)
- Loss of bladder control
- Vision problems
Botox is associated with several less serious but comparatively common side effects:
- Crooked or lopsided smile
- Eyelid droop (ptosis)
- Swelling or pain around the injection site
- Bruising around the injection site
- Headache
- Flulike symptoms, such as fever and chills

Clinicians should inform patients that these symptoms warrant reporting but do not necessitate immediate medical attention. Clinicians should follow up with patients one to two weeks after treatment to evaluate efficacy and discuss any suspected side
effects.
For more information on general safety issues and possible complications or side effects associated with Botox and other medications derived from BoNT-A, refer to the FDA’s postmarket drug safety circular.
When to Repeat Botox Treatment
One of the answers most eagerly sought by Botox treatment candidates is exactly how long before Botox works.
Most Botox candidates see some effect within two to five days following injection, with full effects typically appearing within one or two weeks. Depending on the patient, injection site(s), dosing, and other important factors,
Botox’s paralytic effects may last three to six months before dissipating (often on the lower end of this scale). Botox’s pain-reducing effects may persist for longer.
Accordingly, most Botox patients can repeat treatment
two or three times per year if desired and indicated. Clinicians should treat each repeat treatment as a new patient encounter, taking care to verify that the patient remains a suitable candidate and has not developed any relevant
medical issues that contraindicate Botox treatment.
Alternatives to Botox: What to Know
For years, Botox had the market for clinical botulinum toxin A to itself, earning billions for Allergan.
That’s no longer the case. Botox has indeed spawned several imitators — cosmetic and therapeutic formulations
derived from BoNT-A and BoNT-B. While Botox remains far and away the most popular treatment, it’s not the only choice for clinicians and patients anymore. And it’s highly likely that additional options will enter the marketplace
in the coming years.
Botox competitors are mostly beyond the scope of a guide about Botox itself, but clinicians do need to understand the most popular.
-
Dysport® (abobotulinum toxin type A). Dysport has greater diffusive potential than Botox. This makes it more suitable for treatment of larger muscle groups but may lessen the duration of its effects,
necessitating more frequent treatments. Dysport also contains small concentrations of lactose and milk proteins, a concern for patients with dairy sensitivities.
-
Xeomin® (incobotulinum toxin type A). Of all the differences between Botox and Xeomin, the absence of complexing proteins is among the most significant. This feature eliminates the need to refrigerate
Xeomin before use, making the treatment process easier for practitioners and reducing patient discomfort immediately following injection. Research suggests that Xeomin may be more clinically potent than Botox (requiring
a lower dose to achieve similar outcomes) and may be suitable for patients resistant to Botox.
-
Jeuveau® (prabotulinum toxin type A). Jeuveau is presently approved only for cosmetic use. Its primary advantage over Botox is its low cost.
-
Neurobloc® (botulinum toxin type B). Neurobloc is indicated for the treatment of a single musculoskeletal condition: cervical dystonia. Because it is only used in hospital settings, its commercial potential
is limited relative to Botox.
- Myobloc® (rimabotulinum toxin type B). Myobloc is indicated for the treatment of two musculoskeletal conditions: cervical dystonia and chronic sialorrhea (excessive saliva production).
Looking Ahead: Botox in the 2020s
It’s no exaggeration to say that Botox has taken the world by storm. Since its clinical potential first became clear in the 1970s, tens of millions of Botox doses have been administered to qualified patients in clinical settings.
Today, well over a dozen FDA-approved cosmetic and therapeutic uses exist for Botox. The medication’s demonstrated power to improve quality of life and well-being — and, in some cases, treat debilitating medical issues
without major surgery — is appreciated by patients and clinicians the world over.
The final chapter of Botox’s story has yet to be written, however. As the growing market for Botox competitors shows, patient and clinician
appetites for safe, effective BoNT-A-derived treatments remain great. And research into the active ingredient’s cosmetic and therapeutic potential remains robust. In the years to come, it is very likely that the U.S. Food and
Drug Administration and other health authorities around the world will approve Botox and similar medications for new uses.
What those new uses are, and how they improve prospective patients’ lives, we can’t yet say
with certainty. What we do know is that Botox has made life better for many millions of patients already while adding valuable new practice areas for thousands of trained clinicians. Peering through his microscope at the tiny,
deadly bacteria responsible for its active ingredient, Dr. van Ermengem could scarcely have imagined such a turn of events.